Abstract
Background: The problem of resistance and intolerance to 2nd generation tyrosine kinase inhibitors (2G TKIs) in patients (pts) with chronic myeloid leukemia (CML) currently remains relevant. Ponatinib has demonstrated a high effectiveness and may be an option in CML pts with resistance or intolerance to available TKIs but the high incidence of vascular adverse events (AEs) limits its broad use. A STAMP (Specifically Targeting the ABL Myristoyl Pocket) inhibitor asciminib has demonstrated a superiority over bosutinib in CML pts previously treated with 2 or more TKIs (Phase III Study). Asciminib is available in Russia under the Managed Access Program (MAP) approved by Novartis.
Aim: to present the first results of the use of asciminib in clinical practice under the MAP program in Russia.
Methods: In total 46 CML pts from 3 Russian clinics were enrolled into the MAP program and received asciminib from September 2019 to June 2021 (1 pt started asciminib in a cinical trial and was transferred to MAP later). We analyzed therapy results of 32 pts who received asciminib for at least 3 months. Patient recruitment, dosing regimen, response monitoring, and toxicity control were performed according to the MAP treatment plan. Complete cytogenetic response (CCyR), major molecular response (MMR) and deep molecular response (MR4) rates were assessed by cumulative incident function (CIF). Differences between the subgroups were considered significant with p value ≤ 0.05 by the Gray's test.
Results: Baseline characteristics: male: 41%; Меdian (Me) age 54 years (range 26-81); Me duration of CML before asciminib was 8 years (range 2-24); 23 pts were in chronic phase (CP) CML, 7 and 2 pts had a history of accelerated phase (AP) and blast crisis (BC), respectively, but were in a second CP at baseline. Nineteen (59%) pts had BCR-ABL mutations, 10 pts (31%) had BCR-ABLt315i clones, 7 (22%) pts had at least two mutations. Eight (25%) pts had additional chromosomal abnormalities (ACAs). Twenty one (66%) pts received ≥4 TKIs, 14 (44%) pts had a history of ponatinib treatment. Me duration of asciminib treatment at the time of analysis was 7 months (range 4-24), 4 (12.5%) pts discontinued asciminib due to lack of efficacy; all pts were alive. The initial asciminib dose was 40 BID in 22 (69%) pts and 200 mg BID in 10 (31%) pts.
CCyR, MMR and MR4 at the time of analysis was achieved in 32% (8/25), 34% (10/29) and 17% (5/30) pts, respectively (considering pts without this kind of response at baseline). The 6 month CIF of CCyR, MMR and MR4 was 27%, 24% and 19%, respectively.
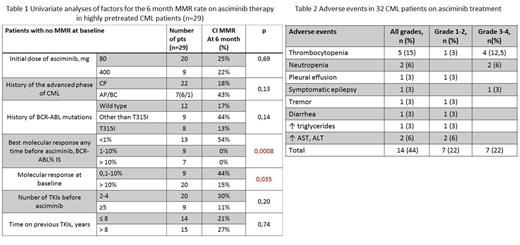
Univariate analysis was performed in 29 pts without MMR at baseline evaluating the following factors of 6 month MMR achievement: initial dose of asciminib, CML phase, presence of BCR-ABL mutations and ACAs, best molecular response on previous TKIs, molecular response at the time of asciminib starting, history of ponatinib treatment, number of TKIs and duration of TKI therapy before asciminib. The duration and number of TKIs, the history of advanced phases, BCR-ABL kinase domain mutations and ACAs did not significantly effect on MMR rate (tab.1). BCR-ABL<1% on previous TKIs (54% vs 0%, p=0.0008, hazard ratio 20.9 (2.6-170)) and BCR-ABL<10% at the time of asciminib start (44% vs 15%, p=0.035, hazard ratio 3.8 (1.05-13.6)) were found as a predictive factors for MMR at 6 month.
Fourteen (44%) of 32 pts had AEs of any grade and 7 (22%) had AEs of grade 3-4 (hematological AEs -6 (19%), non-hematological AE- 1 (3%)) (tab.2).
Conclusion: Asciminib has shown promising efficacy and a good toxicity profile in a cohort of highly pre-treated CML pts and should be considered as a therapeutic option for CML pts resistant or intolerant to other TKIs.
Turkina: Bristol Myers Squibb: Speakers Bureau; Pfizer: Speakers Bureau; Pharmstandart: Speakers Bureau; Novartis Pharma: Speakers Bureau. Lomaia: Novartis: Honoraria; Pfizer: Honoraria; BMS: Honoraria; Pharmstandard: Honoraria. Petrova: Pfizer: Speakers Bureau; Novartis Pharma: Speakers Bureau. Chelysheva: Novartis Pharma: Speakers Bureau; Pharmstandart: Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Pfizer: Speakers Bureau. Gurianova: Pfizer: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal